Boosting Your Health with Mitochondrial Mastery
Have you ever wondered what powers the millions of processes happening in our bodies every single moment? What if I told you there’s an intricate system working non-stop, at a cellular level, to keep you going? And what if there were ways you could directly influence this system to optimize your health and vitality? In this guide, we’ll explore these questions and dive deep into the wonderful world of cell biology today, uncovering how to optimize our mitochondrial function for improved health and longevity.
A Brief History of Mitochondria
Mitochondria have a captivating history. They were first described in the 1850s by a scientist named Richard Altmann who called them ‘bioblasts.’ However, it was not until the late 1960s that scientists began to unravel the complex structure and function of these organelles. The term “mitochondria” was coined by Carl Benda in 1898. Benda was a German cytologist who derived the term from the Greek words “mitos” and “chondros”. “Mitos” means thread, and “chondros” means granule or grain. Benda chose this name based on the thread-like and granular appearance of these structures when viewed under a microscope. However, the name barely scratches the surface of the mitochondrial functions, which range from energy production in the form of ATP to critical roles in cell signaling, cellular differentiation, cell cycle regulation, and even cell death.
- 17th century: The invention of the microscope allows scientists to begin observing cells and their internal structures.
- 1857: Albert von Kölliker observes granular structures within cells that would later be identified as mitochondria.
- 1890: Richard Altmann studies these structures in depth, naming them “bioblasts,” and suggesting they are fundamental to cell function.
- 1898: Carl Benda coins the term “mitochondria,” referring to the Greek words “mitos” (thread) and “chondros” (granule) to describe their appearance.
- 1913: Otto Warburg discovers that mitochondria are the site of cellular respiration.
- The 1950s – 1960s: With the advent of electron microscopy, researchers such as George Palade and Albert Claude reveal the double membrane structure of mitochondria and the internal folds, or cristae, where ATP production occurs.
- The 1960s: Lynn Margulis proposes the endosymbiotic theory, suggesting that mitochondria were once independent organisms that merged with other cells in a symbiotic relationship.
- 1981: The human mitochondrial genome is sequenced, demonstrating that mitochondria have their own DNA separate from the DNA found in the cell nucleus.
- Modern day: Mitochondrial dysfunction is a major focus of research in numerous diseases, and emerging clinical research such as MSC, EVs, and PEMF technology, which utilize our understanding of mitochondria, are being explored.
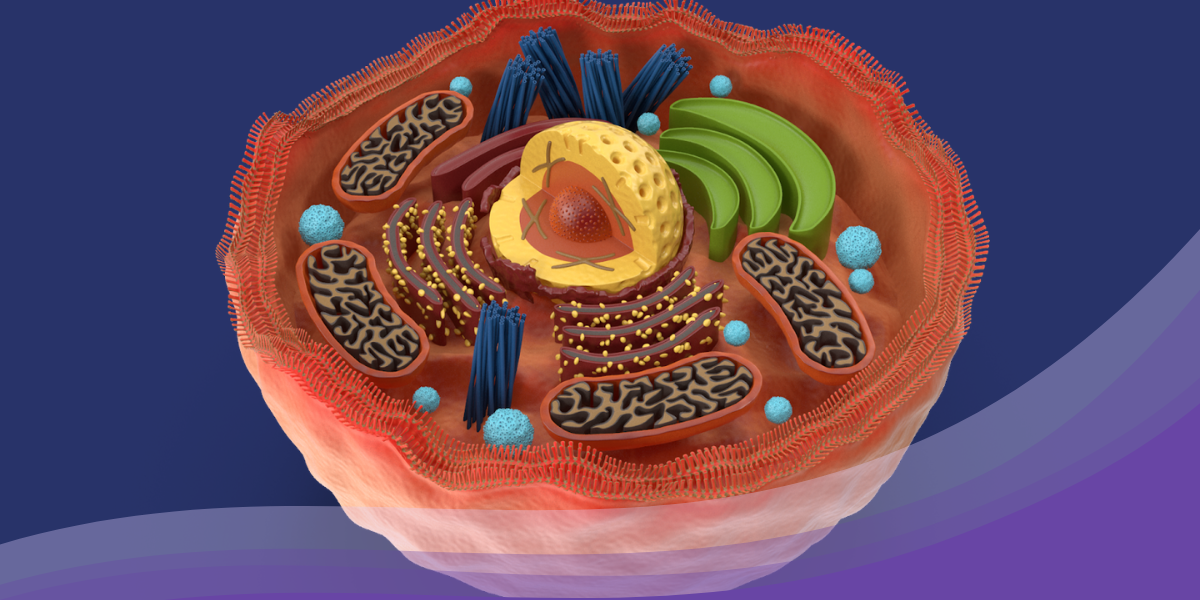
Explaining Mitochondria
The mitochondria are like tiny, organelle-like factories themselves, filled with an army of proteins, lipids, and enzymes. Just like workers in a factory, these components worked together tirelessly, converting the energy from the food the cell ate into a special form that the cells could use. It was as if they were turning plain old apples into supercharged apple juice! Now, the magic ingredient that these mitochondria produced was called adenosine triphosphate, or ATP for short. ATP was like a battery for the cell, providing the energy it needed to perform its daily tasks. It was like the fuel that powered all the metabolic processes inside the cell – everything from thinking and moving to growing and repairing.
But, like any busy factory, the mitochondria faced challenges. They were susceptible to environmental stressors, genetic defects, and even the effects of aging. Sometimes, they would suffer from oxidative damage or mutations in their DNA. These unfortunate events could lead to something called “mitochondrial dysfunction.” It was as if the power lines in the factory got all tangled up, causing chaos and trouble. When mitochondrial dysfunction occurred, it caused a lot of trouble for the cell. The cell’s delicate balance was disrupted, leading to cellular damage, inflammation, and even diseases. It was like the whole factory was thrown into disarray, and everyone was scrambling to fix things.
Mitochondrial Dysfunction
How Dysfunctional Powerhouses of Cells Impact Your Health
The signs of mitochondrial dysfunction can be subtle and varied, given that mitochondria play a role in nearly every cell and system in the body. Here are a few potential signs:
- Fatigue and Weakness: Given that mitochondria are responsible for creating most of the energy your body uses, fatigue is often the first sign of mitochondrial dysfunction.
- Muscle Pain and Weakness: If your muscles don’t have enough energy to work properly, you may experience muscle weakness, pain, or even loss of muscle coordination.
- Neurological Issues: Mitochondrial dysfunction can affect the nervous system, leading to symptoms like seizures, migraines, strokes, or other neurological issues.
- Gastrointestinal Problems: If the mitochondria in your digestive tract aren’t working properly, you may experience symptoms like nausea, bloating, constipation, or diarrhea.
- Heart Problems: Mitochondria are very important in heart muscle cells, so mitochondrial dysfunction can cause heart disease.
- Diabetes: Mitochondria play a role in insulin resistance, so dysfunction can potentially lead to diabetes.
- Hearing and Vision Loss: Mitochondrial dysfunction can also impact the nerves related to hearing and vision, leading to the loss of these senses.
If you suspect mitochondrial dysfunction, it’s essential to seek medical advice. Mitochondrial diseases are complex, and management typically involves a multidisciplinary approach that may include nutritional therapies, physical therapy, avoidance of environmental toxins, and possibly medications or supplements to support cellular energy production. Increasing the quality of your nutrition, engaging in regular physical activity, ensuring you get sufficient quality sleep, and reducing stress can all play a part in supporting mitochondrial health.
1. How does the unique structure of mitochondria contribute to their ability to produce energy?
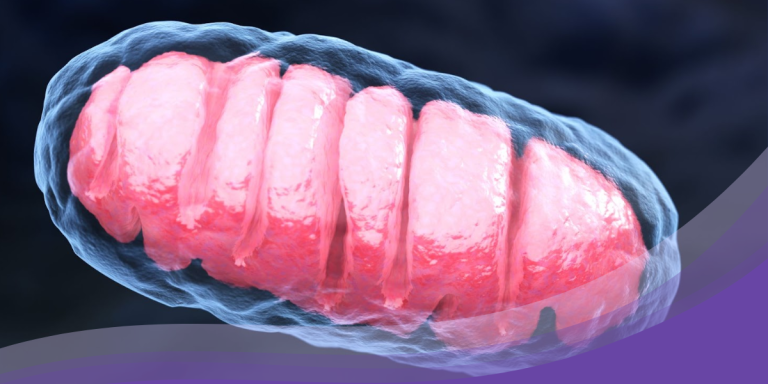
Mitochondria have a distinctive double membrane structure. The outer membrane is relatively permeable and allows the passage of ions and small molecules. The inner membrane, however, is much more selective, housing the electron transport chain and ATP synthase, the key components of the energy-producing process known as oxidative phosphorylation. This membrane is heavily folded into structures called cristae, effectively increasing the surface area for energy production.
2. Can the efficiency of mitochondria be improved, and if so, how can we accomplish that?
Mitochondrial efficiency can indeed be influenced. A balanced diet rich in nutrients, regular exercise, adequate sleep, and stress management can all contribute to maintaining healthy mitochondria. In particular, foods rich in antioxidants can help counteract oxidative damage, while regular physical activity is known to stimulate mitochondrial biogenesis – the creation of new mitochondria. On a cellular level, processes like mitophagy and mitochondrial fusion/fission help maintain a healthy mitochondrial population by clearing out damaged mitochondria and allowing mitochondrial content to be shared and mixed.
3. Do all cells contain the same number of mitochondria, or does it vary depending on the type of cell?
The number of mitochondria per cell can vary greatly depending on the cell’s energy needs. Highly active cells like heart muscle cells, liver cells, and neurons may contain thousands of mitochondria to meet their high energy demands. Conversely, less active cells may contain fewer mitochondria.
4. How can mitochondrial dysfunction influence our overall health, and what might be some less-known conditions associated with it?
Mitochondrial dysfunction can have far-reaching effects on our health because of the central role mitochondria play in energy production, cell signaling, and apoptosis. Beyond the well-known mitochondrial diseases, which are typically severe and often fatal, mitochondrial dysfunction has been implicated in a range of disorders including heart disease, diabetes, neurodegenerative diseases like Parkinson’s and Alzheimer’s, and even some forms of cancer. Aging itself is also associated with a decline in mitochondrial function, potentially contributing to the broad spectrum of age-related diseases.
5. Is it true that mitochondria have their own DNA, separate from our own cellular DNA?
Absolutely, mitochondria do have their own DNA, separate from our cellular DNA. This mitochondrial DNA, or mtDNA, is a tiny circular genome that encodes 13 proteins necessary for energy production. This unique feature hints at the evolutionary origins of mitochondria: they are thought to have been independent organisms that were engulfed by our ancestral cells in a process known as endosymbiosis, eventually becoming an integral part of the cell.
6. How do mitochondria communicate with other parts of the cell, and what happens if this communication is disrupted?
Mitochondria interact with other parts of the cell in several ways, including through physical contact sites, the exchange of lipids and proteins, and the production of signaling molecules. One critical communication pathway is via reactive oxygen species (ROS), which are byproducts of mitochondrial energy production and serve as important signaling molecules. However, when mitochondrial function is compromised, excessive ROS can be produced, leading to oxidative damage and further impairing cellular function. This ‘ROS-induced ROS release’ can lead to a vicious cycle of cellular damage.
7. What are some of the latest research developments in understanding mitochondrial function and its influence on aging and diabetes?
Research into mitochondrial function is a rapidly developing field. In the context of aging and diabetes, a key area of focus is on understanding how mitochondrial dysfunction contributes to these conditions and identifying ways to counteract this. Recent studies have implicated impaired mitochondrial dynamics, increased ROS production, and reduced ATP production in both aging and the development of diabetes. There is also growing interest in the role of mitophagy in these conditions, as this process is often impaired in aging and diabetic tissues, leading to the accumulation of damaged, dysfunctional mitochondria.
One exciting area of research is the use of mesenchymal stem cells (MSCs) and extracellular vesicles (EVs) to improve mitochondrial function. MSC-EVs are shown to contain molecules that can enhance mitochondrial function and help protect against oxidative damage. They also have the unique ability to transfer healthy mitochondria to cells with dysfunctional ones, potentially rejuvenating these cells and improving their function.
Similarly, pulsed electromagnetic field (PEMF) technology, is being investigated for its potential to enhance mitochondrial function. It has been shown to increase the efficiency of mitochondrial respiration and ATP production by activating the production of nitric oxide (NO), an important regulator of mitochondrial function.
8. Can our diet or lifestyle choices impact the health and efficiency of our mitochondria?
Yes, diet and lifestyle can significantly impact mitochondrial health and efficiency. Remember – the power is within you, right down to your cells! Eating a nutrient-rich diet with plenty of antioxidants can help protect against oxidative damage. Foods like fruits, vegetables, nuts, and whole grains are particularly beneficial. Regular physical activity can stimulate the production of new mitochondria and enhance the efficiency of existing ones. Adequate sleep and stress management are also crucial for maintaining mitochondrial health.
9. What role do mitochondria play in the function of our immune system?
Mitochondria play a surprisingly large role in the immune system. They contribute to the immune response in a number of ways, from regulating the energy required for immune cells to function, to controlling the inflammatory response, to even participating in the recognition and response to pathogens. They achieve this through a variety of mechanisms, including ROS signaling, apoptosis, and other forms of cell death.
10. How does the process of mitophagy – the removal of dysfunctional mitochondria – work, and why is it important for maintaining cellular health?
Mitophagy is a specialized form of autophagy, the cellular process that recycles unwanted or damaged cellular components. In mitophagy, damaged mitochondria are encapsulated in a double-membrane structure called an autophagosome, which then fuses with a lysosome, an organelle filled with enzymes that break down the contents of the autophagosome. Mitophagy is crucial for maintaining cellular health because it prevents the accumulation of dysfunctional mitochondria that could produce excessive ROS and release harmful substances into the cell. By removing these problematic organelles, mitophagy helps to maintain a healthy population of mitochondria, ensuring efficient energy production and reducing the risk of cell death.